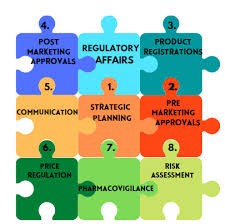
Regulatory Strategy Development
Creating a plan to navigate regulatory requirements for drug development and approval.
Regulatory Submissions
Preparing and submitting documentation to regulatory bodies like the FDA (U.S. Food and Drug Administration), EMA (European Medicines Agency), and other global agencies. This includes Investigational New Drug (IND) applications, New Drug Applications (NDA), and Marketing Authorization Applications (MAA).

Clinical Trial Applications
Assisting with the submission of Clinical Trial Applications (CTA) to regulatory authorities to gain approval to start clinical trials.
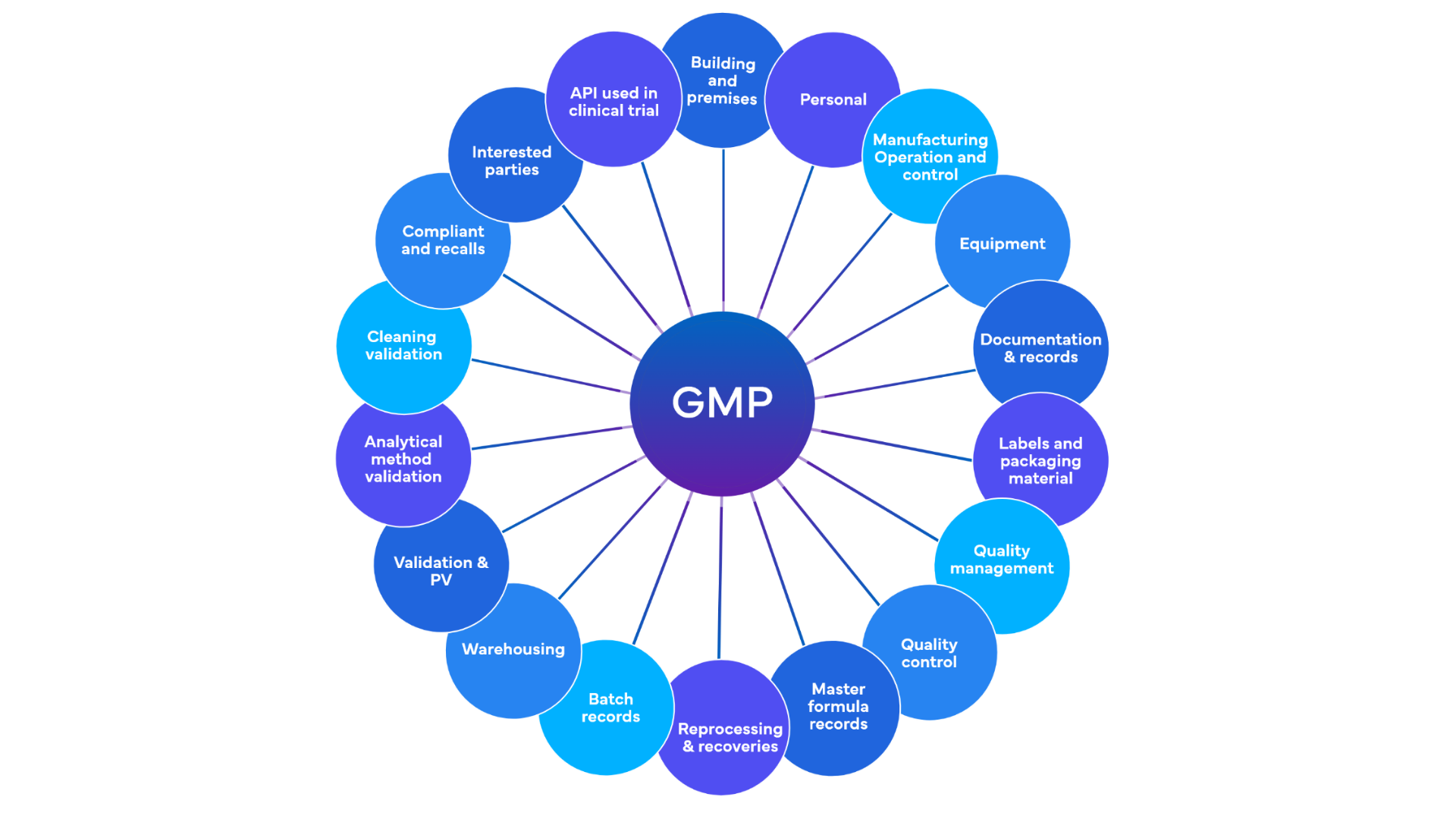
Compliance Consulting
Ensuring that pharmaceutical companies comply with all applicable regulations, including Good Manufacturing Practices (GMP), Good Clinical Practices (GCP), and Good Laboratory Practices (GLP).

Labeling and Packaging Review
Ensuring that pharmaceutical companies comply with all applicable regulations, including Good Manufacturing Practices (GMP), Good Clinical Practices (GCP), and Good Laboratory Practices (GLP).

Regulatory Intelligence
Ensuring that drug labeling and packaging meet regulatory standards and are compliant with legal requirements.

Post-Market Surveillance
Monitoring the safety and efficacy of a drug after it has been released on the market, including adverse event reporting and risk management.
Training and Education
Providing training programs for staff on regulatory requirements and best practices.

Quality Assurance and Auditing
Conducting audits and assessments to ensure compliance with regulatory standards.
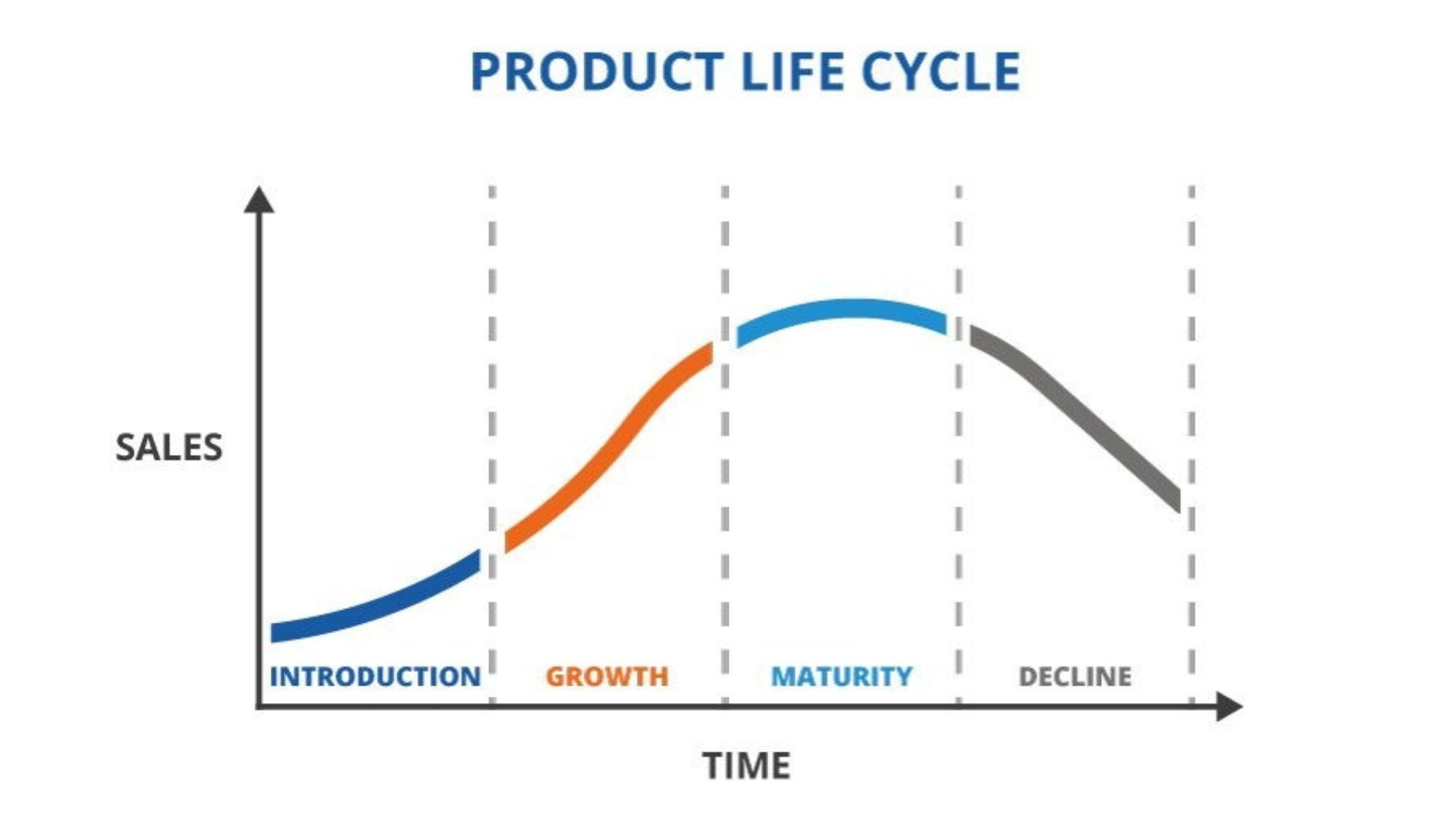
Product Lifecycle Management
Supporting the product throughout its lifecycle, from development to post-marketing, ensuring ongoing compliance with regulatory requirements.
